Research
Biomass Conversion Project | Ammonia synthesis project
Biomass Conversion Project
Solid Brønsted Acid Catalyst
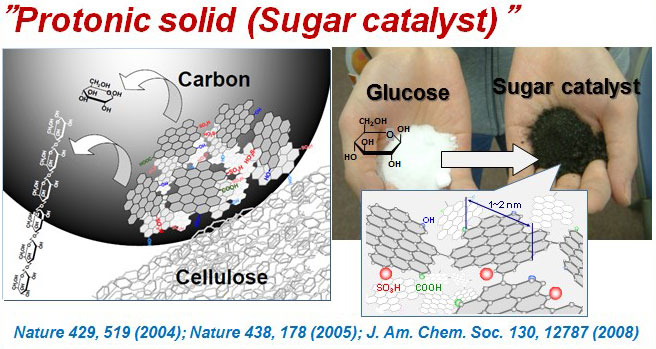
Liquid acids such as sulfuric acid and hydrochloric acid are examples of Brønsted-acid catalysts, and are essential for the production of chemical resources; however, the neutralization procedures required at the completion of reactions with these catalysts entail a significant environmental burden, including large quantities of salts produced as byproducts. In contrast, solid-form Brønsted-acid catalysts offer several advantages, which include ease of recovery by separation and no need for neutralization procedures; however, they typically suffer from significantly inferior catalytic performance compared to that of liquid acids. To address these challenges, we have created carbon-based solid acids, a revolutionary new type of strong Brønsted acid—in solid form—based on a novel design concept. These materials are monolithic structures that consist of nanoscale carbon sheets to which high densities of sulfo, carboxyl, and hydroxyl groups are bound. These catalysts are applied to a variey of reactions, including hydrolysis, esterification, and hydration, where they exhibit catalytic performance equivalent or superior to that of sulfuric acid. Carbon-based solid acids are easily separated from the products, and their performance does not degrade, even after more than 1,500 hours of continuous operation. In addition, we have established a method for the mass-production of carbon-based solid acid catalysts using wood powders as a raw material; efforts to apply these catalysts to commercial production plants are currently in progress. The scientific merit of these materials was recognized by a Scientific American 50 Award (2006), while the practical utility of the research was recognized by a Science and Technology Award (Development category) (2012) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Multifunctional water-tolerant heterogeneous Lewis acid catalysts
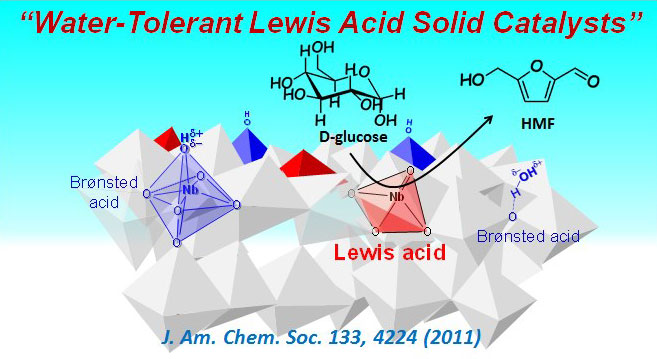
If revolutionary heterogeneous catalysts could be developed for the selective promotion of reactions that consist of multiple steps—such as dehydration, isomerization, oxidation, and reduction—in water, it may be possible to accomplish bulk chemical production from wood and other natural resources, a process that has been difficult to achieve in practical applications. Our group first discovered that transition-metal oxides from group 4 (Ti, Zr) and group 5 (Nb, Ta) can function as Lewis acid catalysts, even in water. Catalytically active sites that are typically deactivated in water are thus made functional, and with these new catalysts we have achieved selectivity—under mild reaction conditions—for a wide variety of complex sugar conversion reactions that could not have been achieved in water with the general-purpose catalysts available in the past. At present, this result has reached the stage of testing to assess suitability for practical applications and has been selected as one of the core topics for Development of production process technologies for chemical products produced from inedible plants, a National Project initiative of the Ministry of Economy, Trade and Industry of Japan.
Ammonia synthesis project

Mass production of ammonia was first made possible by the establishment of the Haber-Bosch process some 100 years ago. Today, worldwide ammonia production exceeds 170 million tons annually and continues to grow. The invention of this process served to ensure a plentiful supply of chemical fertilizers (nitrogen-based fertilizers) essential for food production and today is responsible for supporting a global population of over 7 billion. Moreover, ammonia has become a focus of attention in recent years because it can be liquefied at room temperature under 10 atmospheric pressure, which allows for its use as an easily stored and easily transported energy carrier. This has made the goal of reducing energy consumption in ammonia production even more important than it was before. In research motivated by this context, we have created catalysts based on a new material, 12CaO·7Al2O3 electride (C12A7:e-), which offers a new route for ammonia production with a low environmental burden. Our proposed catalyst material, Ru/C12A7:e–, which consists of Ru nanoparticles fixed in C12A7:e–, facilitates the efficient dissociation of N2 molecules—due to powerful electron injection—and functions as a catalyst for ammonia production at rates that exceed those of traditional catalysts by an order of magnitude, and with half the activation energy. Moreover, because hydrogen poisoning—the greatest disadvantage of Ru catalysts—may be suppressed in these catalysts, their efficiency in ammonia synthesis does not decrease under pressurized conditions. After this result was published in Nature Chemistry, it was reported in publications including Chemical Sciences News in RSC Advances—as well as Japan’s Yomiuri Shimbun and Nikkei Shimbun newspapers—and was recognized as a “Notable Accomplishment” by Japan’s Cabinet Office.


