| Research | Apparatus |
Research(Click here for a research introduction video (about 9 minutes).)
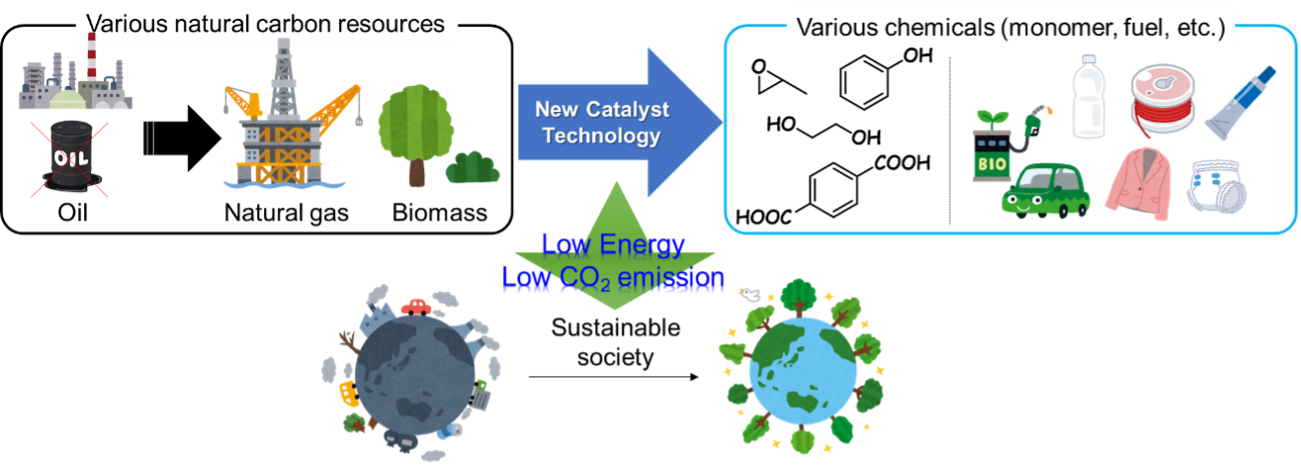
Kamata group will start in April 2023. We aim to contribute to the construction of a sustainable carbon-neutral society through the development of novel catalyst materials and chemical reactions.
Based on the knowledge of inorganic synthesis obtained from research on homogeneous catalysts (polyoxometalates, which are anionic metal oxide cluster molecules), we are challenging the science of new solid catalysts based on crystalline metal oxides. In particular, we aim to develop catalyst technology to produce useful chemicals (monomers, fuels, etc.) from various natural carbon resources such as natural gas and biomass with low energy and CO2 emissions, in sharp contrast to the current chemical processes which are mainly dependent on fossil resources such as petroleum.
v Our recent research works are as follows.
Development of new nanostructure control methods for metal oxides
Nanomaterials exhibit superior properties and unique functions in comparison to their corresponding bulk counterparts. We are developing new nanostructure control methods to create metal oxides with desired compositions and crystal structures.
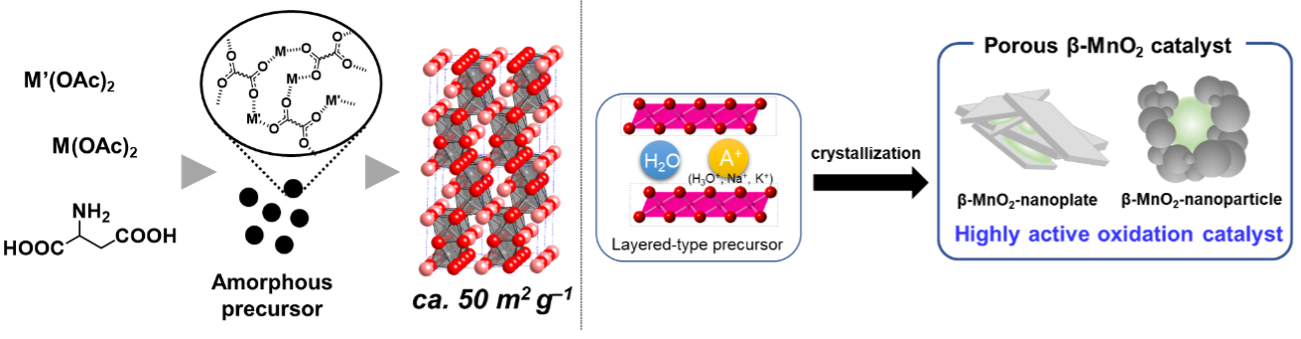
We successfully synthesize crystalline complex oxide nanoparticles with versatile elemental compositions using aspartic acid as a metal dispersant. Various hexagonal perovskite oxides, metal phosphates, and muldokite-type oxides with excellent oxidation catalytic performance are synthesized by sing this “amino acid-aided method” (ACS Appl. Mater. Interfaces
2018, etc.). We also develop template-free synthesis methods of mesoporous β-MnO2 and OMS-1 nanoparticles by a simple method of solid-state transformation of low-crystallinity layered manganese oxide precursors. The pore size and morphology can be controlled by selectinve appropriate reaction conditions, and these mesoporous β-MnO2 and OMS-1 nanoparticles exhibit unique oxidation catalysis due to their structures (ACS Appl. Mater. Interfaces
2020;J. Am. Chem. Soc.
2022, etc.).
Creation of new oxidation catalysts using molecular oxygen as the sole oxidant
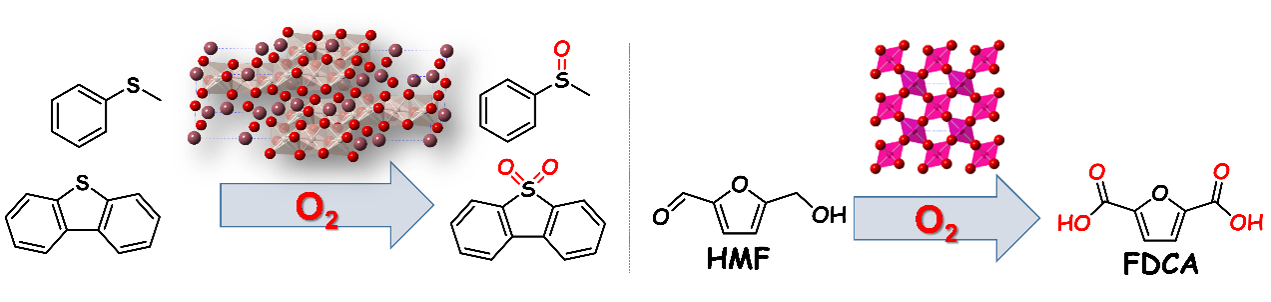
Selective oxidation is an important reaction that accounts for about 30% of industrial organic chemical processes. We are working on the development of new catalyst materials that enable highly difficult selective oxidation reactions using environment-friendly oxidants such as H2O2 and O2.
Focusing on crystalline metal oxides with unique active site structures, we are developing selective oxidation reactions using only O2 as an oxidant. Through joint research with the theoretical calculation group (Prof. Oba Group, TokyoTech), we clarify the relationship between the vacancy formation energy and reactivity of oxygen atoms in metal oxides. On the basis of this concept, we reveal the effectiveness of hexagonal perovskites with high valent B-site metals and β-type manganese dioxide with low vacancy formation energies. Highly difficult selective oxidation reactions including the activation of inert C–H bonds in alkanes, oxygenation of sulfides, plastic monomer synthesis from biomass-derived 5-hydroxymethylfurfural (HMF), and one-pot synthesis are efficiently catalyzed by these new materials (e.g. J. Am. Chem. Soc. 2019).
High functionalization of multicomponent solid catalysts
The combined effect of different elements in the solid likely leads to unique catalytic performance that cannot be achieved with single oxides or homogeneous catalysts. We are developing crystalline complex oxide catalysts in which several molecules are activated in concert on adjacent active sites with different properties (redox, acid-base, etc.).
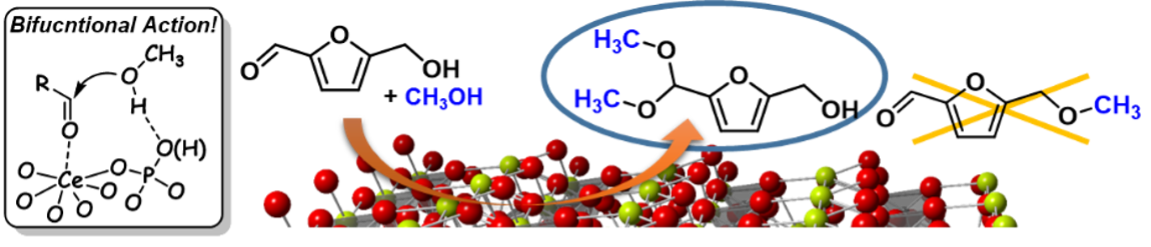
Nanorod-shaped cerium phosphate has uniform Lewis acid and adjacent weak base sites on the solid surface, and this catalyst gives only the acetal derivative for the reaction of biomass-derived 5-hydroxymethylfurfural (HMF) with alcohols in sharp contrast to other homogeneous and heterogeneous acid and/or base catalysts (Chem. Sci. 2017).
We also demonstrated that strontium titanate nanoparticles with high surface area function as acid-base bifunctional catalysts and are effective in the highly efficient synthesis of useful organic compounds (ACS Appl. Mater. Interfaces 2023).
Furthermore, we achieve a highly difficult selective oxidation of methane by adding redox ability to metal phosphates (Catal. Sci.
Technol. 2021; Catal. Sci. Technol. 2023). Nanosized murdochite-type oxide Mg6MnO8 exhibits superior catalytic performance to those of other Mn- and Mg-based oxides for the oxidation of alkylarenes with O2 as the sole oxidant under mild conditions, and the structure of Mg6MnO8 consisting of isolated Mn4+ species located in a basic MgO matrix plays an important role in the present basicity-controlled mechanism of hydrogen atom transfer (ACS Appl. Mater. Interfaces 2022).
These original synthesis technologies can be widely applied to various functional materials. By collaborating with experts in different fields, we are conducting research on “Ambient-temperature oxidative coupling of methane in an electric field” (Prof. Sekine/Prof. Ogo, Waseda Univ.、Prof. Matsumura/Yamaoto, Kyushu Univ.; Chem. Commun. 2019) “Electrocatalysts for alkaline water splitting” (Prof. Yamaguchi/Prof. Sugawara, Tokyo Tech; ACS Appl Energy Mater. 2019, ACS Appl. Energy Mater. 2021, ACS Appl. Energy Mater. 2023).
Apparatus
In addition to the following experimental equipments owned by our group, we can use a large number of equipments through OFC and Collaborative Research Projects.
- Evaporation systems (EYELA N-1110, etc.)
- Spray Dryer (BUCHI Mini Spray Dryer B-290)
- Hydrothermal reactors
- Planetary Mono Mill (FRITSCH P-6)
- Freeze dryer (EYELA FD-1000)
- Low Speed Bench-top Centrifuge (TOMY LCX-100)
- Electrochemical Measurement System (Bio-Logic Science Instruments SP-150e)
- pH Meter (TOA DKK HM-30R)
- Ultrasonic Homogenizer (Yamato LUH150)
- Liquid-phase reactors (TECHNO APPLICATIONS ALHB-80 & DTC-200HZ-3000, SIBATA CP-1000, AS ONE MyBL-100CS)
- Gas-phase reactor (fixed-bed continuous-flow reactor, pulse reactor)
- Electric furnace(NITTO NHK-170AF, AS ONE ROP-001P)
- GC (Shimadzu GC 8A/2025, GL Sciences GC 3210 etc.)
- Single Channel Automated Flash Chromatography System (Yamazen EPCLC-AI-580S)
- Glass overn (BUCHI Glass Oven B-585 Kugelrohr)
- XRD (Rigaku MiniFlex600)
- EDX (Rigaku NEX-DE)
- CATALYST ANALYZER (Microtrac MRB BELCAT II)
- FT-IR (Shimadzu IRSpirit/IRTracer-100)
- UV-Vis (Shimadzu UV-2600)
- TG-DTA(Shimadzu DTG-60H)
- High Throughput Surface Area and Porosity Analyzer (Micromeritics/Shimadzu TristarII Plus 3020)
- Automated Titrator (METTLER TOLEDO EasyPlus)
- Closed glass-circulation system (Makuhari)